A K De Environmental Chemistry Pdf
Magnetite is an iron-oxide mineral that occurs naturally on Earth. Because it is also an important component of many anthropogenic materials (e.g., coal fly ash) and synthetic products (e.g., black toner powders), magnetite can be released to the environment through human activities (1). In PNAS, Maher et al. (2) describe the abundant presence in the human brain of magnetite nanoparticles. Excess use of chemical pesticides in agriculture assisting the accomplishment of agri-food production targets. But, indiscriminate use of chemical pesticide is the serious concern for environment and human, which deteriorating the soil health, nutritional quality of food and increasing resistance in phyto-pathogens and pests. Nanotechnology provided the sustainable solution in this regard.
| Shenandoah Valley Governor's School | |
|---|---|
The SVGS logo is a form of the Sierpinski triangle. | |
| Address | |
, 22939 | |
| Information | |
| Type | Magnet school |
| Established | 1993 |
| School district | Augusta County Public Schools |
| Director | Lee Ann Whitesell |
| Grades | 11-12 |
| Number of students | 175 |
| Color(s) | Teal, purple |
| Website | Shenandoah Valley Governor's School |
The Shenandoah Valley Governor's School is one of Virginia's 18 state-initiated magnetGovernor's Schools. It is a part-time school where 11th and 12th grade students take advanced classes in the morning (receiving their remaining classes from their home high school).
Curriculum[edit]
A K De Environmental Chemistry Pdf 2016
There are two curricula at SVGS:
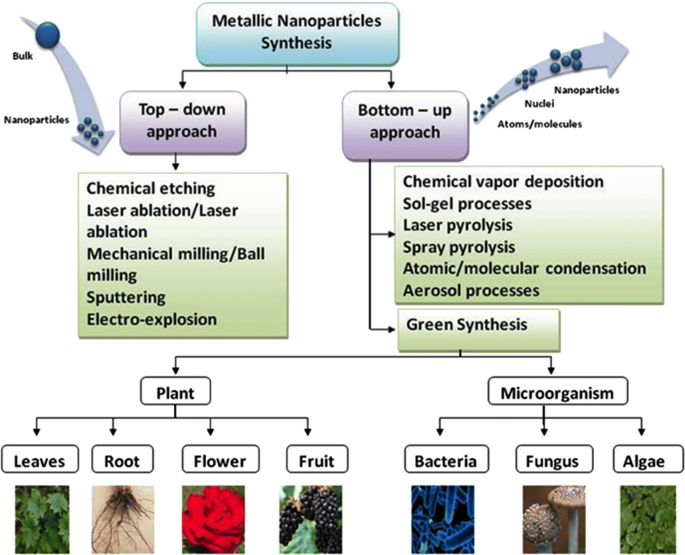
The curriculum for the Math Science and Technology program includes the following college prep and dual enrollment classes: Research and Engineering, Robotics, Advanced Technology, Astrophysics, AP Computer Science, Pre Calculus, AP Calculus, DE Calculus, DE Discrete Mathematics, AP Statistics, DE Molecular Biology, AP Environmental Science, DE Environmental Chemistry, DE Physics, and Modern Physics.
The curriculum for the Arts and Humanities program includes DE Acting I (through JMU), Acting II, DE Introduction to Theatre (through JMU), Advanced Dramatic Theories and Criticism, advanced art classes, DE Humanities 111/112, DE Communication, DE Psychology, and DE The Humanities in Western Culture (through BRCC).
Contributing schools[edit]
Extracurricular activities[edit]
- Electric Vehicle Team
- Robotics Team
- Network Team
- Envirothon Team
- Outreach (with elementary and middle schoolers)
External links[edit]
Coordinates: 38°6′55.8″N78°59′20″W / 38.115500°N 78.98889°W
Chlorinated paraffins (CPs) are complex mixtures of polychlorinated n-alkanes. The chlorination degree of CPs can vary between 30 and 70 wt%. CPs are subdivided according to their carbon chain length into short-chain CPs (SCCPs, C10–13), medium-chain CPs (MCCPs, C14–17) and long-chain CPs (LCCPs, C>17). Depending on chain length and chlorine content, CPs are colorless or yellowish liquids or solids.[1]
Production[edit]
Chlorinated paraffins are synthesized by reaction of chlorine gas with unbranched paraffin fractions (<2 % isoparaffins, <100 ppm aromatics) at a temperature of 80–100 °C.[2] The radical substitution may be promoted by UV-light.[3][1]

- CxH(2x+2) + y Cl2 → CxH(2x−y+2)Cly + y HCl
When the desired degree of chlorination is achieved, residues of hydrochloric acid and chlorine are blown off with nitrogen. Epoxidized vegetable oil, glycidyl ether or organophosphorous compounds may be added to the final product for improved stability at high temperatures.[4][5]

A K De Environmental Chemistry Pdf Online
Commercial products have been classified as substances of unknown or variable composition. CPs are complex mixtures of chlorinated n-alkanes containing thousands of homologues and isomers[6] which are not completely separated by standard analytical methods.[7]
CPs are produced in Europe, North America, Australia, Brazil, South Africa and Asia.[8] In China, where most of the world production capacity is located, 600,000 tons of chlorinated paraffins were produced in 2007.[9] Production and use volumes of CPs exceeded 1,000,000 tons in 2013.[10]
Industrial applications[edit]
Production of CPs for industrial use started in the 1930s.[11] Currently, over 200 CP formulations are in use for a wide range of industrial applications, such as flame retardants and plasticisers, as additives in metal working fluids, in sealants, paints, adhesives, textiles, leather fat and coatings.[12][1]
Safety[edit]
Short-chain CPs are classified as persistent and their physical properties (octanol-water partition coefficient (logKOW) 4.4–8, depending on the chlorination degree) imply a high potential for bioaccumulation. Furthermore, SCCPs are classified as toxic to aquatic organisms, and carcinogenic to rats and mice. Therefore, it was concluded that SCCPs have PBT and vPvB properties and they were added to the Candidate List of substances of very high concern for Authorisation under REACH Regulation.[13] SCCPs (average chain length of C12, chlorination degree 60 wt%) were categorised in group 2B as possibly carcinogenic to humans from the International Agency for Research on Cancer (IARC).[14] In 2017, it was agreed to globally ban SCCPs under the Stockholm Convention on Persistent Organic Pollutants, effective December 2018. However, also MCCPs are toxic to the aquatic environment and persistent; MCCPs in soil, biota, and most of the sediment cores show increasing time trends over the last years to decades; MCCP concentrations in sediment close to local sources exceed toxicity thresholds such as the PNEC.[10]
References[edit]
- ^ abcRossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E. L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack (2006). 'Chlorinated Hydrocarbons'. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2.
- ^Brooke 2009, pp. 4, 23. sfn error: no target: CITEREFBrooke2009 (help)
- ^Lassen 2014, p. 50. sfn error: no target: CITEREFLassen2014 (help)
- ^Kellersohn 1998. sfn error: no target: CITEREFKellersohn1998 (help)
- ^Brooke 2009, p. 5. sfn error: no target: CITEREFBrooke2009 (help)
- ^Tomy 1997, pp. 2764–2765. sfn error: no target: CITEREFTomy1997 (help)
- ^Lassen 2014, p. 30. sfn error: no target: CITEREFLassen2014 (help)
- ^Lassen 2014, pp. 50–51. sfn error: no target: CITEREFLassen2014 (help)
- ^De Boer 2010, p. 8. sfn error: no target: CITEREFDe_Boer2010 (help)
- ^ abJuliane Glüge, Lena Schinkel, Konrad Hungerbühler, Ronan Cariou, Christian Bogdal (2018). 'Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review'(PDF). Environmental Science & Technology. 52 (12): 6743–6760. doi:10.1021/acs.est.7b06459.CS1 maint: multiple names: authors list (link)
- ^Kenne 1996. sfn error: no target: CITEREFKenne1996 (help)
- ^De Boer 2010, p. 9. sfn error: no target: CITEREFDe_Boer2010 (help)
- ^'Candidate List of substances of very high concern for Authorisation: Alkanes, C10–13, chloro (Short Chain Chlorinated Paraffins)'. ECHA. Retrieved 2 January 2020.
- ^IARC 1990, p. 70. sfn error: no target: CITEREFIARC1990 (help)
Sources[edit]

- De Boer J., El-Sayed Ali T., Fiedler H., Legler J., Muir D. C., Nikiforov V. A., Tomy G. T., Tsunemi, K. (2010). The handbook of environmental chemistry 10: Chlorinated paraffins. Berlin: Springer-Verlag. ISBN978-3-642-10760-3.
- Brooke, DM; Crookes, MJ; Merckel, MD (2009). Environmental risk assessment: long-chain chlorinated paraffins, Bristol, UK: Environment Agency.
- 'Chlorinated paraffins'(PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 48: 55–72. 1990. ISBN978-92-832-1248-5. PMID2197463.
- Kellersohn, Thomas (1998). Chlorinated paraffins, in Ullmann’s encyclopedia of industrial chemistry, electronic release, 6th ed. Weinheim: Wiley-VCH.
- Kenne, Kerstin; Ahlborg, Ulf G (1996). Chlorinated paraffins, IPCS, Environmental Health Criteria 181; Geneva: World Health Organization. ISBN9241571810.
- Lassen, Carsten et al. (2014). Survey of short-chain and medium-chain paraffins, Copenhagen: Danish Ministry of Environment, Environmental Protection Agency.
- Tomy, Gregg T. et al. (1997). Quantifying C10-C13 polychloroalkanes in environmental samples by high-resolution gas chromatography/electron capture negative ion high-resolution mass spectrometry, Analytical Chemistry 69, 2764–2765.
Further reading[edit]
- Bayen, Stéphane; Obbard, Jeffrey Philip; Thomas, Gareth O. (2006). 'Chlorinated paraffins: A review of analysis and environmental occurrence'. Environment International. 32 (7): 915–929. doi:10.1016/j.envint.2006.05.009. PMID16814386.
- Cherrie, J. W.; Semple, S. (2009). 'Dermal Exposure to Metalworking Fluids and Medium-Chain Chlorinated Paraffin (MCCP)'. Annals of Occupational Hygiene. 54 (2): 228–35. doi:10.1093/annhyg/mep081. PMID19959560.
- European Chemicals Bureau (2000). European Union Risk assessment report Vol. 4: Alkanes, C10-13, chloro, Luxembourg: Office for Official Publications of the European Community.
- European Chemicals Bureau (2008). European Union Risk assessment report Vol. 81: Alkanes, C10-13, chloro (update), Luxembourg: Office for Official Publications of the European Community.
- European Chemicals Bureau (2005). European Union Risk assessment report Vol. 58: Alkanes, C14-17, chloro (MCCP), Part I-Environment, Luxembourg: Office for Official Publications of the European Community.
- European Commission (2011). European Union Risk assessment report: Alkanes, C14-17, chloro; Addendum to the final report (2007) of the risk assessment - Environment part. Luxembourg: Office for Official Publications of the European Community.
- European Commission (2011). European Union Risk assessment report: Alkanes, C14-17, chloro (MCCP), Part II-Human Health, Luxembourg: Office for Official Publications of the European Community.
- Pellizzato, Francesca; Ricci, Marina; Held, Andrea; Emons, Hendrik (2007). 'Analysis of short-chain chlorinated paraffins: A discussion paper'. Journal of Environmental Monitoring. 9 (9): 924–30. doi:10.1039/b710053a. PMID17726552.
- Tolbert, Paige E. (1997). 'Oils and Cancer'. Cancer Causes & Control. 8 (3): 386–405. doi:10.1023/A:1018409422050. JSTOR3552699. PMID9498901.
External links[edit]

